Dextran Problem
Sugar is an incredible food product found in almost everything we take in our daily lives. In addition to being so abundant in our lives, unfortunately, it does not grow on a tree. The life of sugar starts with sugar beet or sugar cane. To extract and purified this sugar, food technologists and food scientists use many complex steps together. One of these crucial steps is crystallization. This step directly relates to the quality of the sugar (Borji, Borji, & Jourani, 2019). However, high quality is not something that we can achieve so easily. We face problems at this point. For example, the presence of some particles in the sugar solution may affect sucrose crystallization adversely (Borji, Borji, & Jourani, 2019). Among these particles, this article was mainly focused on dextran.
Dextran is a high molecular weight polysaccharide formed by α-(1–6) linked glucose units, with α-(1–3) branch linkages and may contain other branches linkages such as α-(1–2) or α-(1–4) (Bashari et al., 2013). At least 50% to 60% of the linkage must be α-(1-6) to define the molecule as dextran (Coll, Clarke & Roberts, 1974). Their molecular weight ranges from 105-107 and upwards, and usually, there are soluble in water ( high α-(1-6) linkage means higher solubility), but solubility also changes with changing molecular weight (Coll, Clarke & Roberts, 1974).
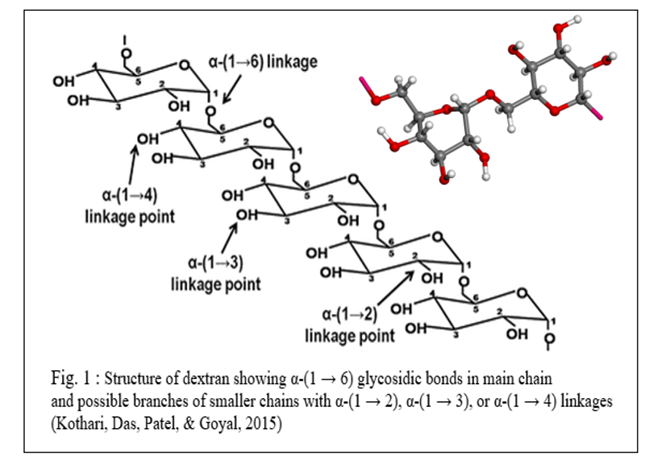
Generally, a healthy sugarcane plant does not contain dextran in substantial amounts; however, it can increase rapidly in damaged cane (before and after harvest) by bacterial contamination (Coll, Clarke & Roberts, 1974). The appearance of dextran in sugar production can cause many problems such as slower filtration rate, increasing viscosity, a rise of sugar loss to molasses, or more separation cycles (Bashari et al., 2013).
Sources and Formation of Dextran
Besides occurring naturally in some foods, dextran is synthesized in sugar solutions by the action of the bacterial enzyme, dextransucrase, on sucrose (Kothari, Das Patel, &Goyal, 2015 and Coll, Clarke & Roberts, 1974). Dextransucrase is produced by LAB (Lactic Acid Bacteria) of genera, viz., Leuconostoc, Streptococcus, Lactobacillus, Pediococcus, and Weissella (Kothari, Das Patel, &Goyal, 2015).
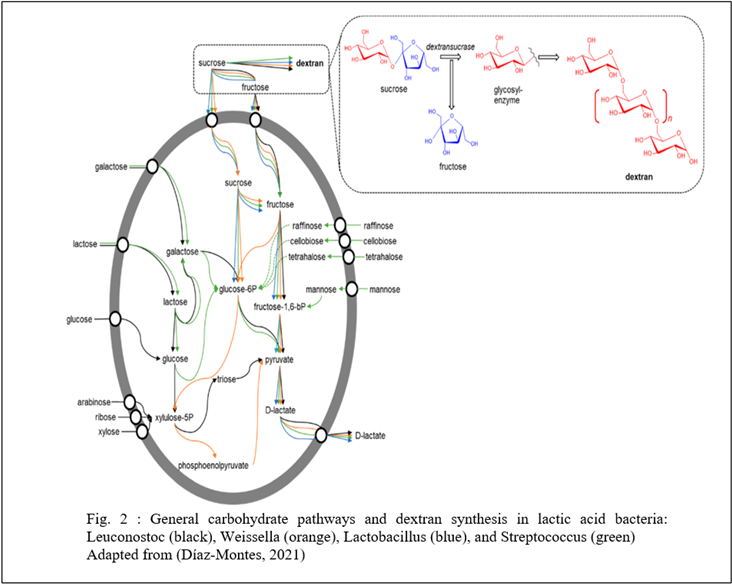
Commonly Leuconostoc mesenteroides and, less commonly, Leuconostac dextranicum (Coll, Clarke & Roberts, 1974) cause this formation. In addition, the nature and degree of the branching of dextran are affected by the bacterium that causes the production of dextran. And this nature directly affects its structure and molecular weight. This variety exists due to how dextran is formed (Coll, Clarke & Roberts, 1974) . Each bacterium, even each strain, produces dextran with its unique structure. However, structural differences seem to have little effect on the problems they cause in sugar production (Coll, Clarke & Roberts, 1974).
Leuconostoc mesenteroides is very abundant in nature, which means contamination with this bacterium is very easy. It can be found in most soils, so the sugar cane is basically in it (Coll, Clarke & Roberts, 1974). It is airborne and can also contaminate standing water (Coll, Clarke & Roberts, 1974). With contamination, this bacterium can grow and produce dextran from any source of sucrose, so a minor infection can create enough dextran in a few days to cause a factory to shut down (Coll, Clarke & Roberts, 1974).
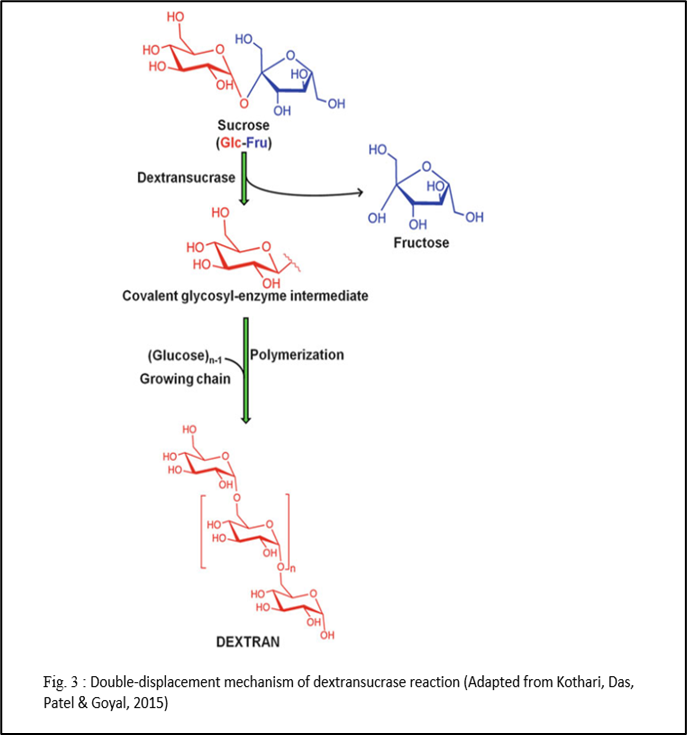
Therefore, it can be understood that increasing dextran amount means reducing the recovery of sucrose during production. Because for dextran production, we need sucrose (Bukhari et al., 2015). From an industrial point of view, dextran also creates processing line problems such as blockage in the filter and pump (Bukhari et al., 2015).
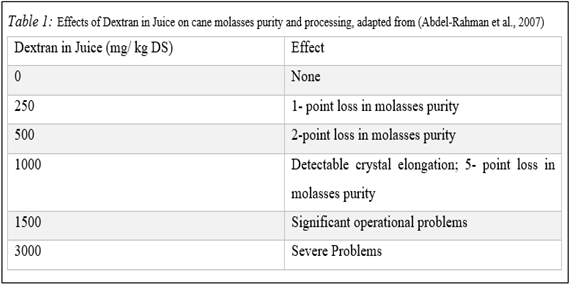
As explained in the above paragraphs, dextran is a big molecule. It can easily increase the viscosity of the juice and create a blockage. Addition to the viscosity problem, dextran can cause low heat exchange, evaporation diminution, blockages in centrifuges (Abraham, 2019; Jiménez, 2009), and a decrease in the efficiency and output of sucrose crystallization, or even stop the crystallization (Abdel-Rahman et al., 2007).
Besides, depending on the molecular mass distribution of dextran (which is ranges from 15 to 2000 kDa in beet and cane juices) present on the plant affects the hydrodynamics of the sugar solution and the characteristics (e.g., size and shape) of the final sucrose crystals (Abraham, 2019). Like mentioned in the beginning paragraph, the damaging effects of high dextran in the technical sucrose solution are evident in the crystallization step (Abdel-Rahman et al., 2007).
Dextran is highly dextrorotatory; actually, even its name came from this ability. In 1874 Scheilber confirmed that this microbial polysaccharide has a positive (dextrorotatory) optical rotation with the empirical formula (C6H10O6)n and therefore named as “dextran.” (Kothari, Das Patel, &Goyal, 2015). This ability can cause false polarization if removed before the test (Jiménez, 2009; Bukhari et al., 2015). High dextran levels also decrease the efficiency of clarification techniques used in polarization determinations (Bukhari et al., 2015). Moreover, because the farmers are primarily paid based on the polarimeter result, there is a requirement for a dextran test in the core laboratory (Bukhari et al., 2015).
Is It Just Bad?
Well, for sugar production, the answer will be yes. However, with a general look, dextran can be used in a good way. Among several EPS (microbial exopolysaccharides), dextran has earned worldwide attention for its biodegradability and biocompatibility properties (Kothari, Das Patel, &Goyal, 2015).
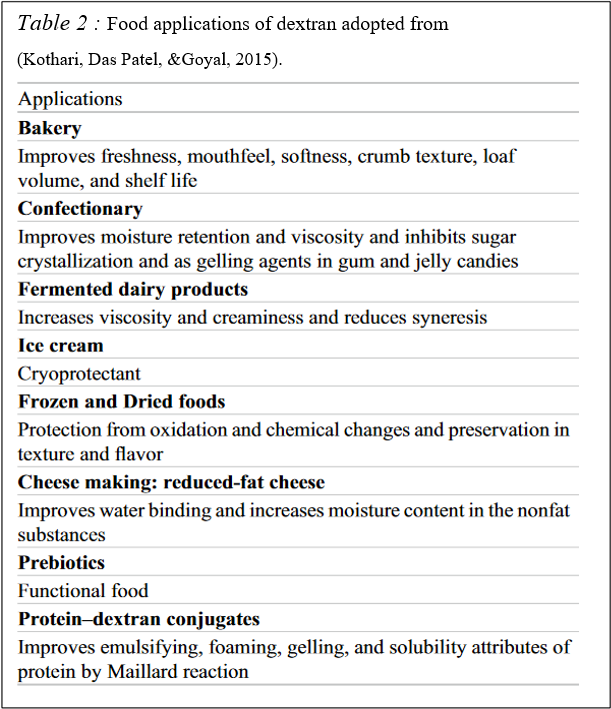
Dextran used many areas such as food, pharmaceutical, biomaterial, photo film manufacturing, and fine chemical industries. Especially in the food industry, it is used as an emulsifier, viscosifier, stabilizer, cryoprotectant, gelling agent, texturing agent, water-binding agent, bio-flocculant, and prebiotic (Kothari, Das Patel, &Goyal, 2015).
CONTENT: Seyda Nur AÇIKGÖZ (Department of Food Engineering, METU)
REFERENCES
Abdel-Rahman, E., Smejkal, Q., Schick, R., El-Syiad, S., & Kurz, T. (2007). Influence of dextran concentrations and molecular fractions on the rate of sucrose crystallization in pure sucrose solutions. Journal of Food Engineering, 84(4), 501–508. https://doi.org/10.1016/j.jfoodeng.2007.06.014
Abraham, K. (2019). Dextran in Sugar Manufacture – Problem Evaluation and Enzyme-Based Mitigation.
Bashari, M., Tounkara, F., Abdelhai, M. H., Lagnika, C., Xu, X., & Jin, Z. (2013). Impact of Dextranase on Sugar Manufacturing and its Kinetic on the Molecular Weights of Remaining Dextran. Sugar Tech, 15(1), 84–93. https://doi.org/10.1007/s12355-012-0195-4
Borji, A., Borji, F. E., & Jourani, A. (2019). Sugar Industry: Effect of Dextran Concentrations on the Sucrose Crystallization in Aqueous Solutions. Journal of Engineering, 2019, 1–6. https://doi.org/10.1155/2019/7987369
Coll, E., Clarke, M. and Roberts, E., 1974. DEXTRAN PROBLEMS IN SUGAR PRODUCTION. In: Proceedings of the … Technical Session on Cane Sugar Refining Research. [online] Science and Education Administration, U.S. Department of Agriculture, 1978, p.92. Available at: https://books.google.ca
Díaz-Montes, E. (2021). Dextran: Sources, Structures, and Properties. Polysaccharides, 2(3), 554–565. https://doi.org/10.3390/polysaccharides2030033
Jiménez, E. R. (2009). Dextranase in sugar industry: A review. Sugar Tech, 11(2), 124–134. https://doi.org/10.1007/s12355-009-0019-3
Kothari, D., Das, D., Patel, S., & Goyal, A. (2015). Dextran and Food Application. Polysaccharides, 735–752. https://doi.org/10.1007/978-3-319-16298-0_66
The information contained in this document reflects only the view of the SuChAQuality project and in no way reflects the European Commission’s opinion for which cannot be held responsible for any use that may be made of the information it contains

